Development of the Arthritis TAS (ATAS) Clinical Governance Framework and Research Governance Framework were two of the earliest actions addressed by the organisation in its 2017–2020 Corporate Strategy.
Clinical Governance
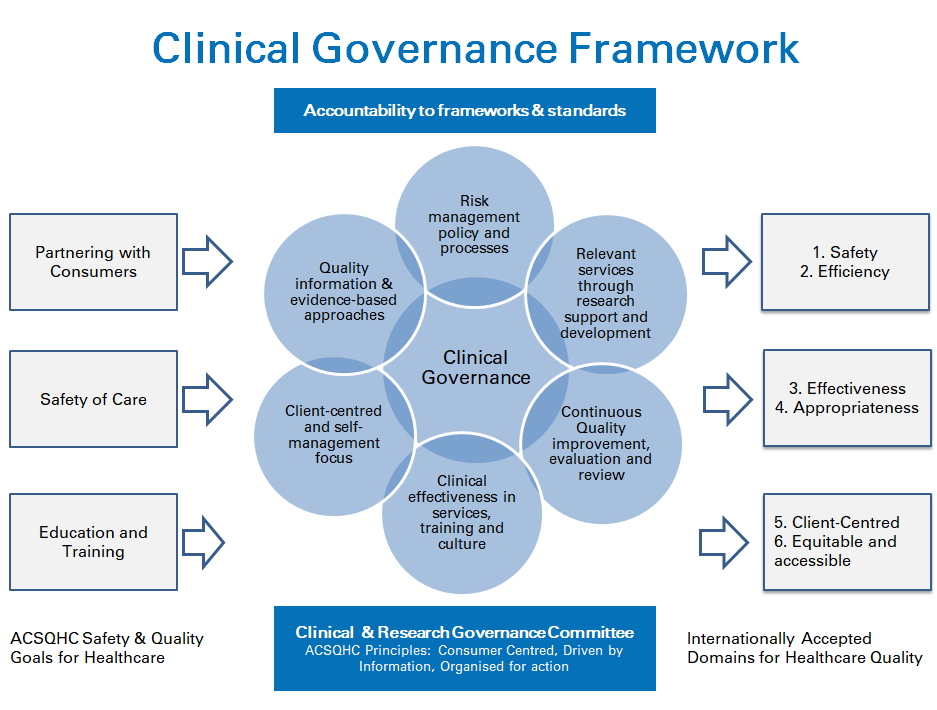
Clinical governance is the system by which the governing body, managers, clinicians and staff share responsibility and accountability for the quality of care, continuously improving, minimising risks and fostering an environment of excellence in care for consumers.
The ATAS Clinical Governance Framework is underpinned by the goals of the Australian Commission for Safety and Quality in Health Care (ACSQHC), with outcomes being the internationally accepted domains for quality in health care.
The Clinical Governance Framework supports Strategy 1: Empowering the individual and consolidates alignment of our health services, such as the Infoline and our Strong exercise classes, to the strategy. The framework ensures that we are following clear policies and processes that are used by hospitals and community health providers to ensure that services are safe, risk is managed, and effectiveness is maximised.
Research Governance Framework
The Research Governance Framework is linked to the Clinical Governance Framework, in particular through the principle of ‘Relevant services and resources through research support and development’. The ATAS Research Management Framework includes grant guidelines to establish a comprehensive understanding of the proposed research context, allocation, priorities and expected research conduct, along with templates for research grant applications, agreements and protocols.
The organisation identified the need for a research framework during strategic planning in 2017, to ensure the robust management of research to be funded where possible through significant ATAS operational surpluses. The Research Management Framework provides clarity regarding eligibility, funding application guidelines, along with transparency in the reporting of research and finances. The framework supports clinical, social and quality improvement projects, ensuring that each align with clinical governance principles, strategic goals and also ATAS vision. Governance components ensure compliance with the Australian Code for the Responsible Conduct of Research, conflict of interest and ethics requirements.
Background
The National Safety and Quality Health Service (NSQHS) Standards introduced the second edition in October 2017. The new edition strengthens clinical governance and focus on communication and comprehensive care, therefore it is an excellent time to introduce research governance that support new quality directions. The ATAS Research Management Framework reflects the second ACSQHC Framework principle to ‘ensure the best available information to support programs and develop new policies and programs that will drive improvement the safety and quality of healthcare’. ATAS is committed to consumer participation in research activities, along with communication of research findings to the community. Successful grant recipients will be asked to give a presentation of research to ATAS consumers and stakeholders. Selection criteria for applications currently focus on ATAS strategic objectives and the international domains for safety and quality.